We’ve released a minor update. It is free for licensed SDScribe™ 2025 users, and you can either update the program when reminded (on program startup), or from our existing customers page.
Among the changes:
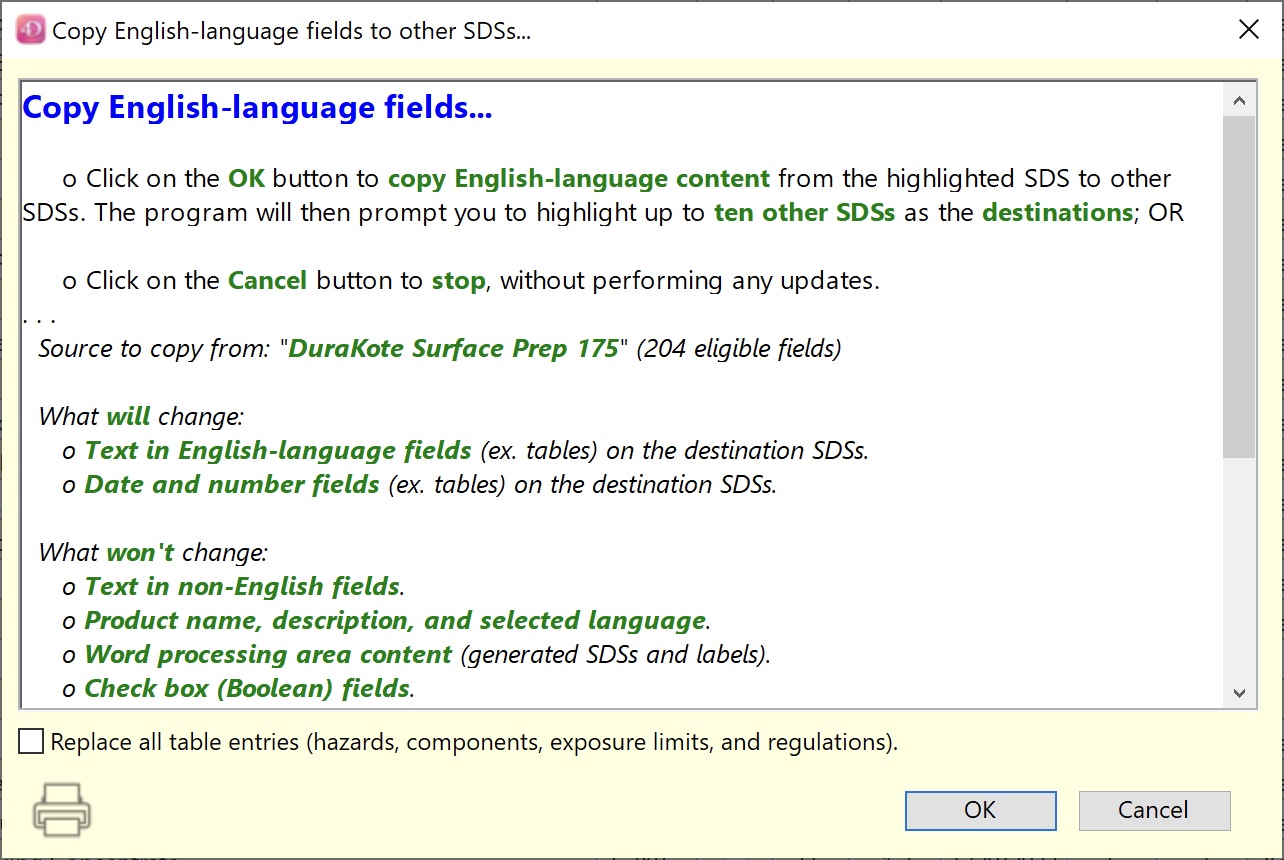
The “Copy English to other SDSs…” function now has an optional check box, to “Replace all table entries (hazards, components, exposure limits, and regulations)” in the destination SDSs. Note that nothing in these existing tables (in the destination SDSs) is updated or retained.
There is an updated import for Substance records, for WGK – Rigoletto (German water hazard class).
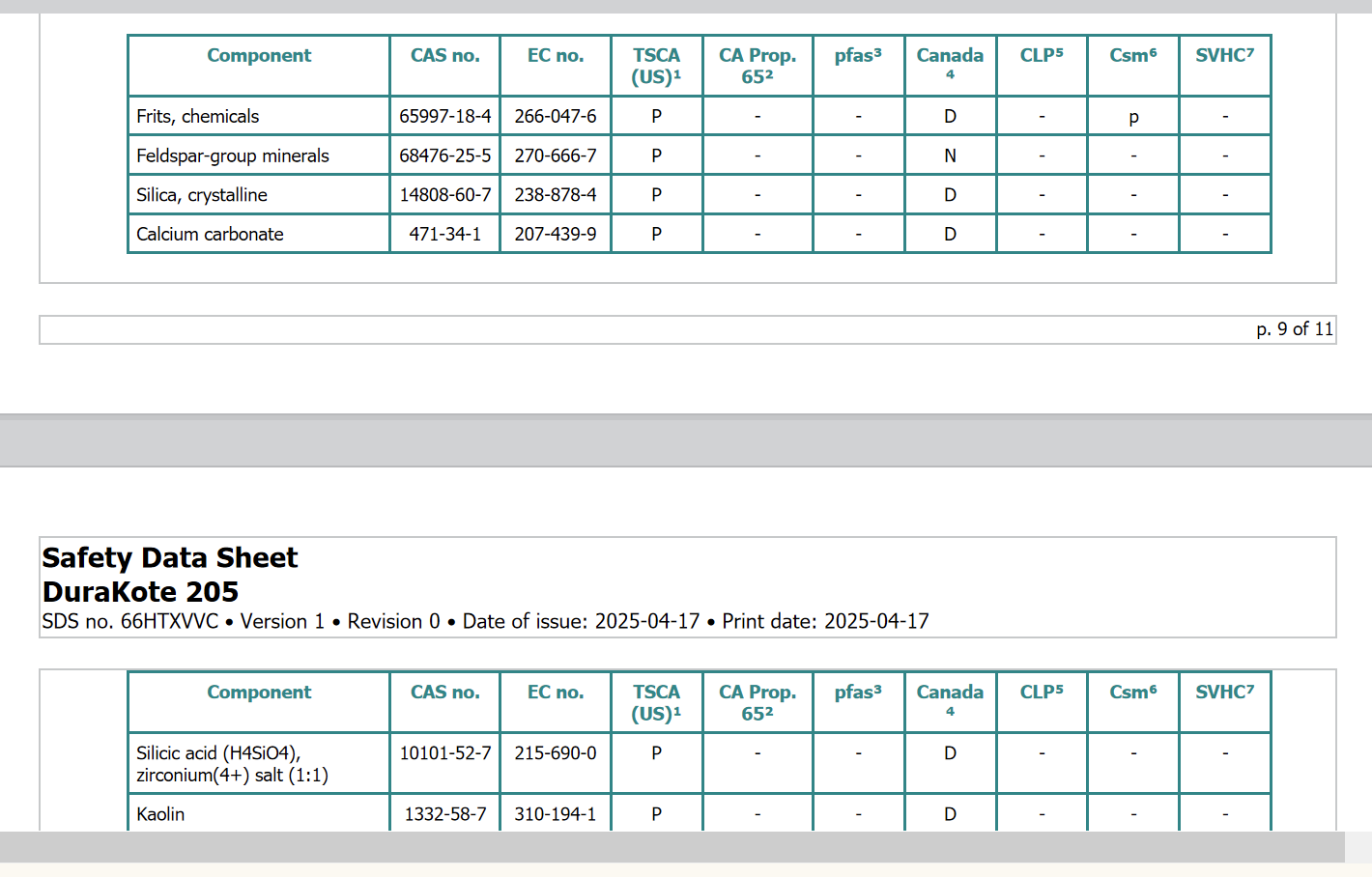
Where either of the regulatory tables in SDS Section 15 cross a page boundary in the generated SDS, the first row should now repeat the table header (column titles).
Miscellaneous minor fixes.